Background
Patients with secondary acute myeloid leukemia (sAML) have poor outcomes compared to those with de novo AML. In 2017, liposomal daunorubicin and cytarabine (CPX-351) was FDA approved for the treatment of adults with newly diagnosed AML with myelodysplasia-related change (AML-MRC) or therapy-related AML (t-AML). In its landmark trial, CPX-351 has displayed significant improvement in overall survival (OS) compared to conventional 7+3 in patients 60-75 years of age with sAML. Gaps remain in the literature regarding the clinical use of CPX-351 in context of the FDA approved label. Here we evaluate real-world outcomes with disease response and molecular monitoring in patients treated with CPX-351.
Methods
Adults who received CPX-351 between September 2017 and December 2019 were identified. The primary endpoint was overall response rate (ORR), defined by complete remission (CR) and CR with incomplete hematologic recovery (CRi) according to the Revised IWG criteria. Additional outcomes of interest included molecular minimal residual disease (MRD) status post induction as measured by next-generation sequencing (NGS), ORR in patients with baseline TP53, and progression-free survival (PFS) in patients with CR/CRi, with and without MRD after induction. Mutations associated with clonal hematopoiesis (TET2, ASXL1, DNMT3A) were excluded from analysis of molecular MRD.
Results
Fifty-four patients were identified with baseline characteristics as shown in Table 1. Overall, the study population was elderly with the median age of 64 [IQR: 60-68], and 13 patients were younger than 60 years old. Six patients developed AML in the setting of a pre-existing myeloproliferative neoplasm (MPN). The most common indication for treatment with CPX-351 was antecedent MDS (42.6%), followed by de novo AML with MDS karyotype (24.1%), therapy-related AML (13%), and antecedent MPN (11.1%). NGS was performed prior to treatment with CPX-351 in all but one patient, and 88.7% had at least one molecular marker that is not identified as one of the mutations associated with clonal hematopoiesis. Most commonly identified molecular markers were TP53 (16/53, 30.2%), RUNX1 (10/53, 18.9%), SRSF2 (8/53, 15.1%), NRAS (7/53, 13.2%), and IDH2 and JAK2 (6/53, 11.3%, each).
Most patients were hospitalized until hematologic recovery. However, 5 patients received induction in the outpatient setting, and an additional 6 patients were discharged early before hematologic recovery. Among the patients who were discharged early or underwent outpatient induction, 81.8% (9/11) were admitted for a complication. There were no deaths associated with outpatient induction. Overall, 46 patients (85.2%) experienced febrile neutropenia and 17 patients (31.5%) had bacteremia. Thirty-day and 60-day mortality were 9.3% and 14.8%, respectively.
The ORR was 54%, and the response rates observed in patients who were younger vs older than 60 years were similar (41.7% vs. 57.9%, p=0.508). In patients who achieved a remission after induction, 56% (14/25) were MRD positive by NGS. Among those who had TP53 mutation at baseline, 14 were available for response assessment after induction. The ORR in this subgroup was 57% (8/14) and all but 3 (63%) were MRD negative by NGS. Consolidation with allogeneic transplant was performed in 18 patients (33%).
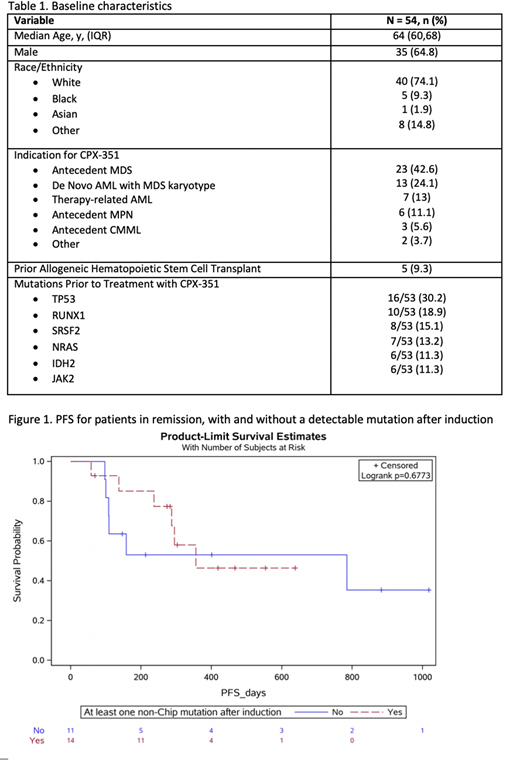
Median OS was 10.4 mos. Median OS was similar for patients older or younger than 60 years (p=0.76). For patients achieving a CR/CRi, median OS had not been reached at the time of analysis but was significantly improved compared to those with refractory disease (6.1 mos, p=0.0007). Median OS or PFS did not differ significantly (p=0.68) based on MRD negativity (Figure 1).
Conclusion
This analysis demonstrates comparable response rates to the landmark trial (54% in our analysis vs. 47.7%). Outpatient induction and/or early discharge was safe and feasible in appropriately selected patients. While this analysis is limited by the small sample size, CPX-351 appeared effective in populations that were not included in the published randomized studies, such as patients below the age of 60 years old and those with antecedent MPN. Remission rates and MRD clearance was high among TP53 mutants. A considerable number of patients who achieved a remission remained MRD positive by NGS, but this did not impact PFS. Future studies should evaluate the impact of molecular MRD and allele frequency to further guide treatment.
Koprivnikar:Alexion: Speakers Bureau; BMS: Speakers Bureau; Novartis: Speakers Bureau; Amgen: Speakers Bureau. McCloskey:Takeda: Consultancy, Honoraria, Speakers Bureau; Novartis: Speakers Bureau; Abbvie: Speakers Bureau; Amgen: Consultancy, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Jazz: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal